Effective probiotics strains for
adults and infants health.
인체유래 비피더스균
SEARCH
인체유래 비피더스균
Probiotics Raw materials
WTAT IS M-16V?
Bifidobacterium breve is one of the predominant species in the infant gut and are widely recognized for its beneficial roles in maintaining infant health. B. breve M-16V has emerged as one of the best studied clinically effective probiotic strains that exert positive effects, particularly in infants, to support healthy growth and promote well-being.
WHY M-16V?
Proven track record of safety
and clinical efficacies
Effective at promoting gut microbial colonization and protecting against health disorders in infants.
Used in >120 NICUs of hospitals in Japan, Australia, New Zealand, and Singapore to support healthy growth of low birth weight infants
SPECIFICATIONS

Human-Residential Bifidobacteria
Isolated from healthy infant in 1963

Evidence-based Safety
Genomic, toxicological, and clinical studies

Clinically Proven
Supported by > 90 scientific studies

Regulatory Approved
FDA notified GRAS status for foods and infant use in 2013

Quality Assured
FSSC22000, HACCP, HALAL

Long History
Safe use in food products for more than 40 years
STABILITY
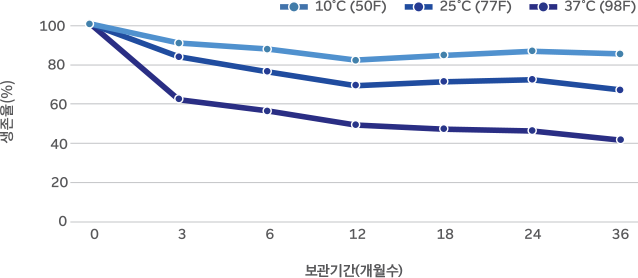
36-month real-time stability study on M-16V powder
M-16V is highly stable due to Morinaga’s unique culturing method and advanced production technology. Survival rate above 50% (based on 37°C, 24 months)
Probiotics Raw materials
WHAT IS BB536?
Bifidobacterium longum subsp. longum BB536 is one of the most well-established, clinically effective probiotic strains that confers numerous profound beneficial effects on humans. BB536 possesses a proven track record of safety and clinical efficacy in improving gastrointestinal, immunological and infectious conditions, as demonstrated in more than 180 scientific studies.
WHY BB536?
Well-documented human probiotic strain
Wide range of beneficial effects
Has been incorporated in various products and widely marketed
SPECIFICATIONS

Human-Residential Bifidobacteria
Isolated from healthy infant in 1969

Evidence-based Safety
Genomic, toxicological, and clinical studies

Clinically Proven
Supported by >180 scientific studies

Regulatory Approved
FDA notified GRAS status in 2009,
Japan FOSHU status in 1996

Quality Assured
FSSC22000, HACCP, HALAL

Long History
Safe use in food products for more than 40 years
STABILITY
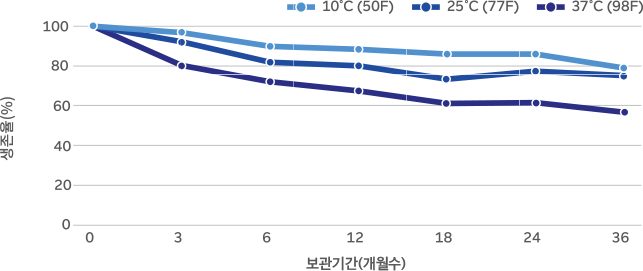
36-month real-time stability study on BB536 powder
BB536 is highly stable due to Morinaga’s unique culturing method and advanced
production technology. Survival rate above 50% (based on 37°C, 24 months)
Probiotics Raw materials
WTAT IS B-3?
Bifidobacterium breve B-3 is an unique probiotic strain, developed by focusing on the relationship between gut microbiota and metabolic syndrome. B-3 possesses an attractive effect in maintaining healthy body weight and ultimately improving one’s lifestyle.
WHY B-3?
Effective at maintaining healthy living
Backed with highly-quality clinical data
Highly specialized in weight management
SPECIFICATIONS

Human-Residential Bifidobacteria
Isolated from healthy infant in 2002

Evidence-based Safety
Genomic, toxicological, and clinical studies

Scientifically Backed Probiotics
Supported by scientific studies

Quality assured
FSSC22000, HACCP, HALAL
STABILITY
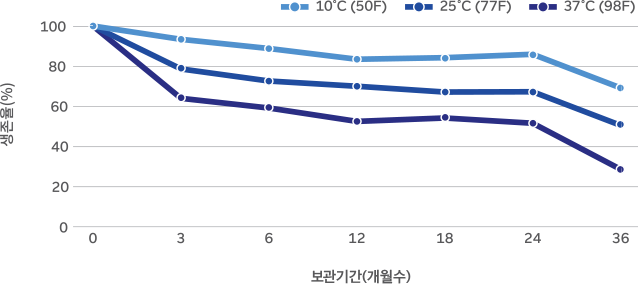
36-month real-time stability study on B-3 powder
B-3 is highly stable due to Morinaga’s unique culturing method and advanced production technology. Survival rate above 50% (based on 37°C, 24 months)
Probiotics Raw materials
WTAT IS M-63?
Bifidobacterium longum subsp. infantis M-63 is unique among gut bacteria in its immense capacity to utilize human milk oligosaccharide (HMOs), the component that is highly abundant in human breast milk. HMOs offer no direct nutritive value for infants, but they function in shaping the infant intestinal microbiota with life-long impacts.
WHY M-63?
High capability on HMOs utilization
Safety and efficacy backed with numerous studies
Superior colonization potential and well-adapted to intestinal environment of humans, especially infants.
SPECIFICATIONS

Human-Residential Bifidobacteria
Isolated from healthy infant in 1963

Evidence- based Safety
Genomic, toxicological, and clinical studies

Suitable for Infant Use
Supported by scientific studies

Quality Assured
FSSC22000, HACCP, HALAL
STABILITY
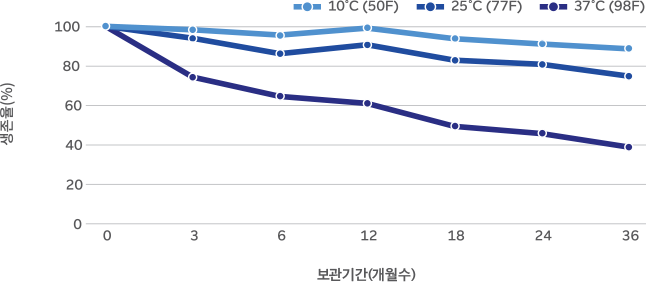
36-month real-time stability study on M-63 powder
M-63 is highly stable due to Morinaga’s unique culturing method and advanced production technology. Survival rate above 50% (based on 37°C, 24 months)